Lab: Acids and Bases
Virtual Lab
Active
Step 3: Create a Model Solution of pH =6 with M...

Chemistry, 16.12.2020 02:50 Wanna14ever
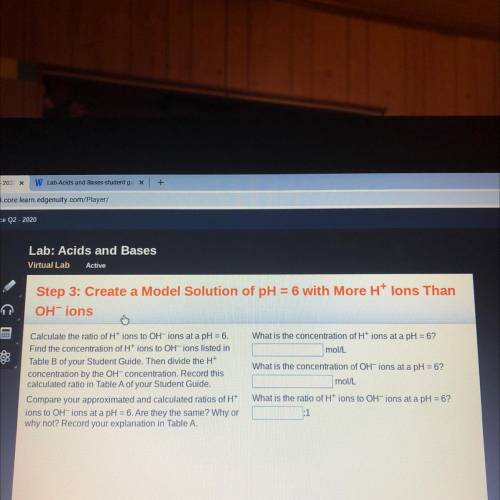
Lab: Acids and Bases
Virtual Lab
Active
Step 3: Create a Model Solution of pH =6 with More Ht lons Than
OHT ions
Calculate the ratio of Ht ions to OH ions at a pH=6.
Find the concentration of Ht ions to OHT ions listed in
Table B of your Student Guide. Then divide the Ht
concentration by the OHT concentration. Record this
calculated ratio in Table A of your Student Guide.
Compare your approximated and calculated ratios of H
ions to OH ions at a pH =6.Are they the same? Why or
why not? Record your explanation in Table A.
What is the concentration of Ht ions at a pH= 6?
mol/l.
What is the concentration of OH ions at a pH =6?
mollL
What is the ratio of Ht ions to OH
ions at a pH6?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1
You know the right answer?
Questions

Mathematics, 13.01.2020 21:31






Mathematics, 13.01.2020 21:31


History, 13.01.2020 21:31

Mathematics, 13.01.2020 21:31


History, 13.01.2020 21:31

Biology, 13.01.2020 21:31

Mathematics, 13.01.2020 21:31

Mathematics, 13.01.2020 21:31


History, 13.01.2020 21:31


Mathematics, 13.01.2020 21:31

History, 13.01.2020 21:31



