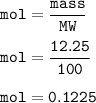Chemistry, 15.12.2020 14:00 royaltyy6533
A scientist completes a thermal decomposition reaction. 12.25g of calcium carbonate, CaCO3, is heated
and forms calcium oxide, Cao, and carbon dioxide, CO2.
a. Write a balanced chemical equation to demonstrate this reaction. Include state symbols.
b. Calculate the number of moles of calcium carbonate that is thermally decomposed in this
reaction.
Ok

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Where are each of the three particles located within the atom?
Answers: 1

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1
You know the right answer?
A scientist completes a thermal decomposition reaction. 12.25g of calcium carbonate, CaCO3, is heate...
Questions





SAT, 16.10.2020 15:01

Mathematics, 16.10.2020 15:01





Mathematics, 16.10.2020 15:01

Physics, 16.10.2020 15:01



Social Studies, 16.10.2020 15:01

English, 16.10.2020 15:01



History, 16.10.2020 15:01

Mathematics, 16.10.2020 15:01