Chemistry, 15.12.2020 01:00 jamieric0324
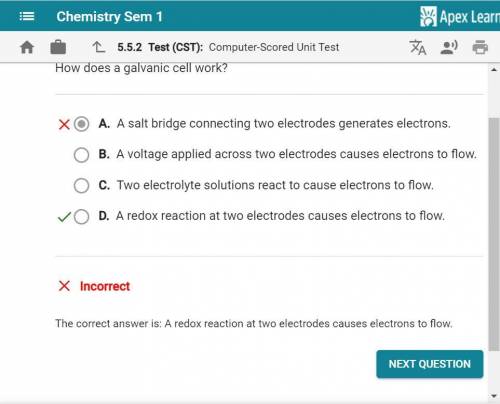
How does a galvanic cell work? A. A voltage applied across two electrodes causes electrons to flow. B. A salt bridge connecting two electrodes generates electrons. O C. A redox reaction at two electrodes causes electrons to flow. D. Two electrolyte solutions react to cause electrons to flow.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2


Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3
You know the right answer?
How does a galvanic cell work? A. A voltage applied across two electrodes causes electrons to flow....
Questions

Mathematics, 02.12.2021 22:00


Mathematics, 02.12.2021 22:00



Mathematics, 02.12.2021 22:00

Advanced Placement (AP), 02.12.2021 22:00

Chemistry, 02.12.2021 22:00

Mathematics, 02.12.2021 22:00

Arts, 02.12.2021 22:00

Social Studies, 02.12.2021 22:00

Computers and Technology, 02.12.2021 22:00


Social Studies, 02.12.2021 22:00

Mathematics, 02.12.2021 22:00





Social Studies, 02.12.2021 22:00