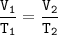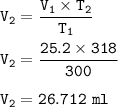Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 07:00
Under what conditions will a gas be most likely to exhibit the ideal gas properties predicted by the ideal gas law? 1)high pressures and high temperature, because particles are forced closer together with higher kinetic energy, so intermolecular forces of attraction are weaker 2)high pressure and low temperature, because particles are forced closer together and moving slower, so the volume of the particles is less significant 3) low pressure and high temperature, because particles are spread farther apart and moving faster, so the intermolecular forces of attraction are weaker 4)low pressure and low temperature, because particles are spread farther apart with lower kinetic energy, so the volume of the particles is less significant
Answers: 2

Chemistry, 23.06.2019 07:00
4. glenn andrews recently bought a motorcycle for $3,950. if he had to pay 6% sales tax on the bike, what was the total cost of the motorcycle?
Answers: 1

Chemistry, 23.06.2019 14:30
An atom of element x has one more shell of electrons than an atom of beryllium, but it has one less valance electron than beryllium. which element is x
Answers: 1
You know the right answer?
3. Decomposition of a sample of KCIO3(s) produced 25.2 mL of 020, at a temperature of
27°C and a pr...
Questions


History, 03.08.2019 10:00

Mathematics, 03.08.2019 10:00

History, 03.08.2019 10:00

Social Studies, 03.08.2019 10:00


History, 03.08.2019 10:00

Mathematics, 03.08.2019 10:00

Mathematics, 03.08.2019 10:00

History, 03.08.2019 10:00




Mathematics, 03.08.2019 10:00





Social Studies, 03.08.2019 10:00