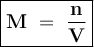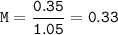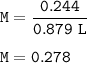Chemistry, 13.12.2020 22:20 harleyandpope90
Calculate the molarity of the two solutions.
The first solution contains 0.350 mol of NaOH in 1.05 L of solution.
molarity:
The second solution contains 14.3 g of NaCl in 879 mL of solution.
molarity:

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1


Chemistry, 23.06.2019 05:30
Elizabeth has two separate samples of the same substance. sample is in the liquid state, and the other is in the solid state. the two samples most likely differ in which property?
Answers: 1
You know the right answer?
Calculate the molarity of the two solutions.
The first solution contains 0.350 mol of NaOH in 1.05...
Questions

English, 11.03.2021 04:40

Arts, 11.03.2021 04:40

Mathematics, 11.03.2021 04:40


Arts, 11.03.2021 04:40

Mathematics, 11.03.2021 04:40

Mathematics, 11.03.2021 04:40



English, 11.03.2021 04:40

Mathematics, 11.03.2021 04:40

Biology, 11.03.2021 04:40

Biology, 11.03.2021 04:40

Mathematics, 11.03.2021 04:40

Mathematics, 11.03.2021 04:40





Mathematics, 11.03.2021 04:40