
Chemistry, 13.12.2020 04:40 jadeochoa4466
For the following reaction, calculate how many moles of each product are formed when 4.05 g of water is used. 2H20 →2H2 + O2

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 22:30
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1

Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1
You know the right answer?
For the following reaction, calculate how many moles of each product are formed when 4.05 g of water...
Questions



Spanish, 07.04.2021 22:40

Mathematics, 07.04.2021 22:40

Mathematics, 07.04.2021 22:40


Chemistry, 07.04.2021 22:40


Chemistry, 07.04.2021 22:40



Mathematics, 07.04.2021 22:40

Mathematics, 07.04.2021 22:40


Spanish, 07.04.2021 22:40


History, 07.04.2021 22:40

Mathematics, 07.04.2021 22:40


English, 07.04.2021 22:40

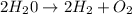
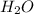 is used to form 2 moles of
is used to form 2 moles of 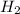 and 1 mole of
and 1 mole of 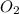
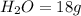 , 1 mole of
, 1 mole of 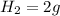 , and 1 mole of
, and 1 mole of 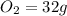 .
.

