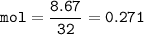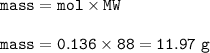Fe(s) + S(s) → FeS(s)
In one experiment, 7.62 g of Fe are allowed to react with 8.67 g of S.
...

Chemistry, 12.12.2020 23:50 lclaudettecarte4720
Fe(s) + S(s) → FeS(s)
In one experiment, 7.62 g of Fe are allowed to react with 8.67 g of S.
What is the limiting reagent, and what is the reactant in excess?
Calculate the mass of FeS formed.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 23.06.2019 01:00
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

You know the right answer?
Questions













English, 21.04.2020 20:38



Mathematics, 21.04.2020 20:38


Mathematics, 21.04.2020 20:38


Chemistry, 21.04.2020 20:38