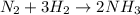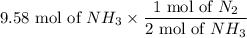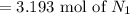Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

You know the right answer?
For the reaction N2 + 3H2 - 2NH3, how many moles of nitrogen are required to produce 9.58 mol of amm...
Questions

Mathematics, 22.01.2021 01:50

Mathematics, 22.01.2021 01:50


Mathematics, 22.01.2021 01:50

Mathematics, 22.01.2021 01:50

Spanish, 22.01.2021 01:50



English, 22.01.2021 01:50

Mathematics, 22.01.2021 01:50

Mathematics, 22.01.2021 01:50

Physics, 22.01.2021 01:50

Arts, 22.01.2021 01:50

English, 22.01.2021 01:50



Computers and Technology, 22.01.2021 01:50

Mathematics, 22.01.2021 01:50