
Chemistry, 12.12.2020 17:10 Jackpumpkin
Sodium carbonate reacts with silver nitrate according to the following balanced equation: Na2CO3 (s) + 2 AgNO3 (aq) → Ag2CO3 (s) + 2 NaNO3 (9) If 3.60 g of Na2CO3 is allowed to react with 5.14 g of AgNO3, what mass of Na2CO3 will remain at the end of the reaction?
a) 2.01 g
b) 1.54 g
c) 0.423 g
d) 0.0150 g
e) 0g

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1


Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1
You know the right answer?
Sodium carbonate reacts with silver nitrate according to the following balanced equation: Na2CO3 (s)...
Questions


Mathematics, 10.11.2020 23:20

Mathematics, 10.11.2020 23:20


English, 10.11.2020 23:20


Mathematics, 10.11.2020 23:20


Business, 10.11.2020 23:20

Mathematics, 10.11.2020 23:20

Mathematics, 10.11.2020 23:20

Mathematics, 10.11.2020 23:20

Social Studies, 10.11.2020 23:20

Arts, 10.11.2020 23:20

Arts, 10.11.2020 23:20

Mathematics, 10.11.2020 23:20

Computers and Technology, 10.11.2020 23:20

Biology, 10.11.2020 23:20

Mathematics, 10.11.2020 23:20

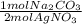 = 0.0151 mol Na₂CO₃
= 0.0151 mol Na₂CO₃

