Chemistry, 12.12.2020 17:10 mistycascaden
Lithium hydroxide is used in alkaline batteries. Calculate the molarity of a solution prepared by dissolving 1.495 moles of LiOH in enough water to give a final volume of 750. mL.
1) 1.99 M
2) 1.50 M
3) 1.12 M
4) 0.502 M
5) 0.00199

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:00
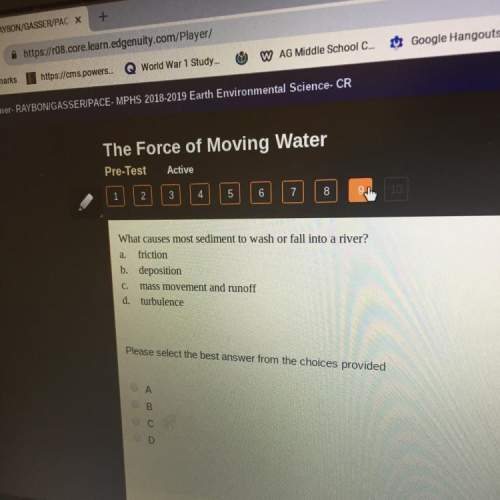
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 23.06.2019 02:00
As light moves from one material into the next, which of the following affects how much the light waves will refract, or bend? angle at which the ray strikes the medium color of the material density of the material temperature of the light wave
Answers: 2

You know the right answer?
Lithium hydroxide is used in alkaline batteries. Calculate the molarity of a solution prepared by di...
Questions


Mathematics, 07.06.2021 22:10

Mathematics, 07.06.2021 22:10

Computers and Technology, 07.06.2021 22:10

English, 07.06.2021 22:10

English, 07.06.2021 22:10

Mathematics, 07.06.2021 22:10



English, 07.06.2021 22:10

Mathematics, 07.06.2021 22:10

Mathematics, 07.06.2021 22:10

History, 07.06.2021 22:10

Chemistry, 07.06.2021 22:10

Mathematics, 07.06.2021 22:10

Mathematics, 07.06.2021 22:10

Mathematics, 07.06.2021 22:10


Biology, 07.06.2021 22:10

Computers and Technology, 07.06.2021 22:10