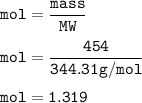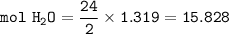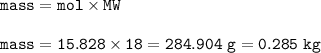Chemistry, 12.12.2020 17:00 miguegen6225
454 grams of isomaltitol, C12H24O11, was combusted in a furnace completely in an excess oxygen. The products of this reaction are water and carbon dioxide. What is the theoretical yield (in kilograms) of water produced from this reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
1) these are barrel shaped microtubules in most animal cells, that organize the spindles during cell division
Answers: 1

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 23.06.2019 03:30
In chemistry, the type of an atom (what element it is) is determined by: a) the number of protons it contains in its nucleus.b) the number of neutrons it contains in its nucleus.c) the number of protons it has in a cloud around the nucleus.d) the number of neutrons it has in a cloud around the nucleus.e) the number of electrons it exchanges with its neighbors.
Answers: 1
You know the right answer?
454 grams of isomaltitol, C12H24O11, was combusted in a furnace completely in an excess oxygen. The...
Questions



Medicine, 26.05.2020 21:01



Computers and Technology, 26.05.2020 21:01

History, 26.05.2020 21:01




Biology, 26.05.2020 21:01

History, 26.05.2020 21:01

Mathematics, 26.05.2020 21:01