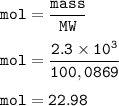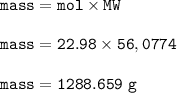Chemistry, 12.12.2020 17:00 jet0120996
When 2.3 × 10^3 g of CaCO3 are heated, the actual yield of CaO is 1.09 × 10^3g. What is
the percent yield?
1. 51.0643
2. 54.084
3. 53.0474
4. 84.5827
5. 59.4924
6. 93.7005
7. 49.4244
8. 78.3748
9. 44.6193
10. 72.7638
Answer in units of %.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 20:00
Listenbase your answer to the question on the information below.nuclear radiation is harmful to living cells, particularly to fast-growing cells, such as cancer cells and blood cells. an external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body.cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. one hospital keeps a 100.0-gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment.which choice represents the correct product for the beta decay of the co-60? fe-60ni-60fe-61ni-61
Answers: 2
You know the right answer?
When 2.3 × 10^3 g of CaCO3 are heated, the actual yield of CaO is 1.09 × 10^3g. What is
the percent...
Questions

English, 03.12.2020 08:40


Social Studies, 03.12.2020 08:40

Mathematics, 03.12.2020 08:40

History, 03.12.2020 08:40



Mathematics, 03.12.2020 08:40




History, 03.12.2020 08:40

Physics, 03.12.2020 08:40

Mathematics, 03.12.2020 08:40


History, 03.12.2020 08:40


Mathematics, 03.12.2020 08:40

Mathematics, 03.12.2020 08:40