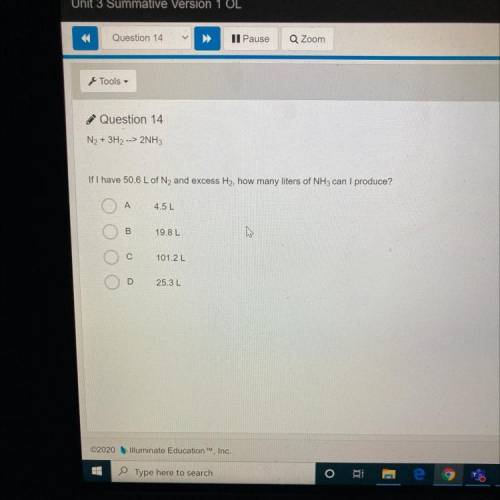
N2 + 3H2, -> 2NH3
If I have 50.6 L of N2 and excess H2, how many liters of NH3 can I produc...

Chemistry, 12.12.2020 16:00 greatsavagebeast
N2 + 3H2, -> 2NH3
If I have 50.6 L of N2 and excess H2, how many liters of NH3 can I produce?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2
You know the right answer?
Questions



Chemistry, 23.04.2021 20:20


Mathematics, 23.04.2021 20:20


Social Studies, 23.04.2021 20:20


Mathematics, 23.04.2021 20:20

English, 23.04.2021 20:20





Mathematics, 23.04.2021 20:20


Mathematics, 23.04.2021 20:20



Mathematics, 23.04.2021 20:20



