
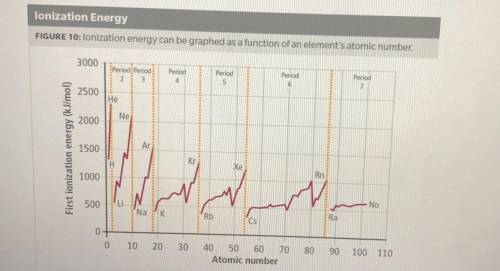
1. How does ionization energy change with atomic number? Use evidence from the graph to support your claim.
2. How does ionization energy change across a period and down a group on the periodic table? Use evidence from the graph to support your claim.
3. What describes an effect on ionization energy when moving down a group? Select all correct answers.
A. The ionization energy increases down a group
B. The ionization energy decreases down a group
C. The valence electrons are in energy levels farther from the nucleus
D. The shielding effect is less

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 22:30
Why is it possible for different microorganisms to extract energy not only from carbohydrates and other biological molecules but from a large variety of substances?
Answers: 1

Chemistry, 23.06.2019 02:00
What can be done to make a solid solute dissolve faster in a liquid solvent?
Answers: 1
You know the right answer?
1. How does ionization energy change with atomic number? Use evidence from the graph to support your...
Questions

Biology, 31.07.2019 15:00


Business, 31.07.2019 15:00



Arts, 31.07.2019 15:00

Arts, 31.07.2019 15:00

Arts, 31.07.2019 15:00




Biology, 31.07.2019 15:00


Mathematics, 31.07.2019 15:00

Business, 31.07.2019 15:00







