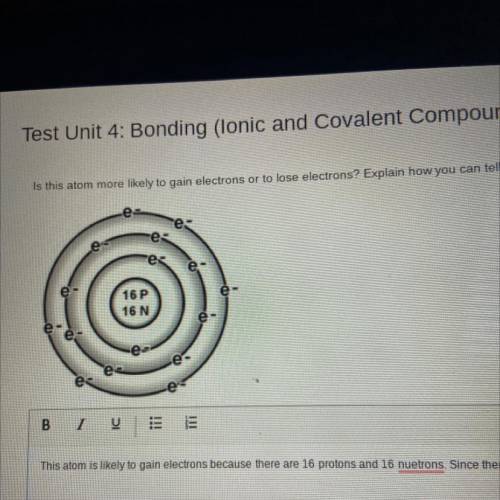
Is this atom more likely to gain electrons or to lose electrons? Explain how you can tell
e
e...

Chemistry, 11.12.2020 01:50 eazywalters
Is this atom more likely to gain electrons or to lose electrons? Explain how you can tell
e
e-
e е
es
e е
16P
16 N
e es
Please help

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:20
1. suppose a reaction mixture, when diluted with water, afforded 300 ml of an aqueous solution of 30 g of the reaction product malononitrile [ch2(cn)2], which is to be isolated by extraction with ether. the solubility of malononitrile in ether at room temperature is 20.0 g/100 ml, and in water is 13.3 g/100 ml. what weight of malononitrile would be recovered by extraction with (a) three 100-ml portions of ether and (b) one 300-ml portion of ether? suggestion: for each extraction, let x equal the weight extracted into the ether layer. in part (a), the concentration in the ether layer is x/100 and in the water layer is (30 x)/300; the ratio of these quantities is equal to k 20/13.3.
Answers: 2

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3
You know the right answer?
Questions

Chemistry, 09.06.2021 21:10

Arts, 09.06.2021 21:10


Social Studies, 09.06.2021 21:10


English, 09.06.2021 21:10

Mathematics, 09.06.2021 21:10

Mathematics, 09.06.2021 21:10



Mathematics, 09.06.2021 21:10

Mathematics, 09.06.2021 21:10

Chemistry, 09.06.2021 21:10


Mathematics, 09.06.2021 21:10

Mathematics, 09.06.2021 21:10

History, 09.06.2021 21:10

Mathematics, 09.06.2021 21:10




