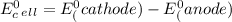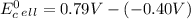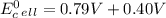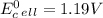Chemistry, 05.02.2020 07:52 heartykwarteng12
Choose the overall cell potential for this redox reaction.
cd + hg22+ → cd2+ + 2hg. given: cd2+ + 2e− → cd(s) –0.40 and hg22+ + 2e− → 2hg(l) 0.79
a.) –0.19 v
b.) 0.19 v
c.) 0.91 v
d.) 1.19 v

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:20
Complete the table for ion charge based upon their losing or gaining electrons in the outer shell. (use the periodic table as necessary.) group most likely ionic charge # of valence electrons i +1 ii +2 iii +3 iv +4 or -4 v -3 vi -2 vii -1 viii 0
Answers: 2

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1
You know the right answer?
Choose the overall cell potential for this redox reaction.
cd + hg22+ → cd2+ + 2hg. giv...
cd + hg22+ → cd2+ + 2hg. giv...
Questions

History, 22.04.2020 23:59


Mathematics, 22.04.2020 23:59

Physics, 22.04.2020 23:59



History, 22.04.2020 23:59


Biology, 22.04.2020 23:59


Social Studies, 22.04.2020 23:59



History, 22.04.2020 23:59

English, 22.04.2020 23:59

History, 22.04.2020 23:59