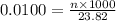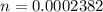Determine the amount of sodium hypochlorite in Bleach you tolerated a 23.82 ml sample of 0.0100 M KIO cuboid with a solution of Na squared S squared O cubid of unknown concentration. The endpoint was observed to occur at 12.38 ml. How many moles of KIO cubid wewe titrated

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 17:20
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2
You know the right answer?
Determine the amount of sodium hypochlorite in Bleach you tolerated a 23.82 ml sample of 0.0100 M KI...
Questions


Chemistry, 20.05.2021 02:10



History, 20.05.2021 02:10




Mathematics, 20.05.2021 02:10

Mathematics, 20.05.2021 02:10

Mathematics, 20.05.2021 02:10




Chemistry, 20.05.2021 02:10

History, 20.05.2021 02:10

Mathematics, 20.05.2021 02:10


Mathematics, 20.05.2021 02:10

Mathematics, 20.05.2021 02:10

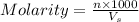
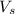 = volume of solution in ml
= volume of solution in ml