
Chemistry, 10.12.2020 09:50 byrdkellykellybyrd
Arrange the aqueous solutions from the most acidic to the most basic, at 25 C.
Acidic
[OH | = 1x 10^-7 M
[OHT|=1× 10^-9 M
[H"] = 1 x 10^-3 M
pH = 11

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 22.06.2019 23:00
If two identical atoms are bonded,what kind of molecule is formed
Answers: 1
You know the right answer?
Arrange the aqueous solutions from the most acidic to the most basic, at 25 C.
Acidic
[OH | =...
[OH | =...
Questions




Mathematics, 09.09.2020 18:01

Mathematics, 09.09.2020 18:01



Mathematics, 09.09.2020 18:01

English, 09.09.2020 18:01

Chemistry, 09.09.2020 18:01


Mathematics, 09.09.2020 18:01






English, 09.09.2020 18:01


World Languages, 09.09.2020 18:01

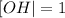 x
x 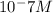
![[H^+] =](/tpl/images/0969/1465/99ddf.png)
 ×
× 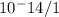 x
x 
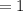 x
x ![pH = - log [H^+]](/tpl/images/0969/1465/04033.png)
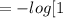 x
x 

