
Chemistry, 10.12.2020 06:20 jonathon3957
URGENT PLEASE HELP!
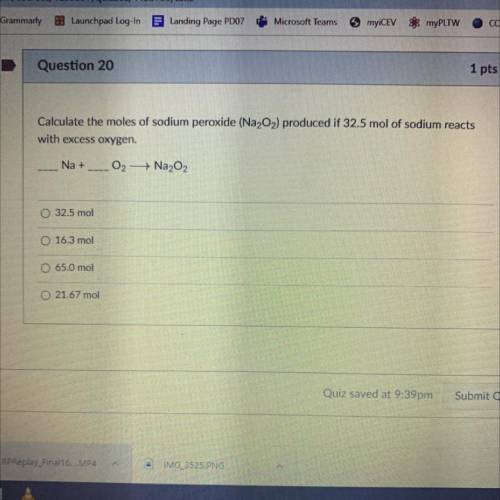
Calculate the moles of Sodium Peroxide (Na2O2) produced if 32.5 mol of sodium reacts with excess oxygen.
__Na+__O2—>Na2O2
A. 32.5 mol
B. 16.3 mol
C. 65.0 mol
D. 21.67 mol
and why is it one of the answer above?

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1
You know the right answer?
URGENT PLEASE HELP!
Calculate the moles of Sodium Peroxide (Na2O2) produced if 32.5 mol of sodium r...
Questions


Mathematics, 02.10.2020 14:01

Arts, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01


Mathematics, 02.10.2020 14:01

History, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01

Social Studies, 02.10.2020 14:01




Mathematics, 02.10.2020 14:01


Computers and Technology, 02.10.2020 14:01

English, 02.10.2020 14:01

History, 02.10.2020 14:01

English, 02.10.2020 14:01


Mathematics, 02.10.2020 14:01



