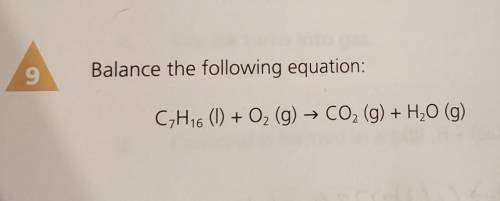Balance the following equation: CH16 (1) + O2 (g) → CO2 (g) + H20 (g)
...


Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
How much energy moves onto the next level, in an energy pyramid
Answers: 1

Chemistry, 22.06.2019 01:00
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
Questions


Mathematics, 26.03.2021 03:50

Mathematics, 26.03.2021 03:50


Mathematics, 26.03.2021 03:50

Biology, 26.03.2021 03:50

Mathematics, 26.03.2021 03:50

Mathematics, 26.03.2021 03:50

Business, 26.03.2021 03:50

Geography, 26.03.2021 03:50

Mathematics, 26.03.2021 03:50

Mathematics, 26.03.2021 03:50

Advanced Placement (AP), 26.03.2021 03:50

History, 26.03.2021 03:50

Business, 26.03.2021 03:50


Mathematics, 26.03.2021 03:50


History, 26.03.2021 03:50