
Chemistry, 10.12.2020 01:30 kyriebarnes143
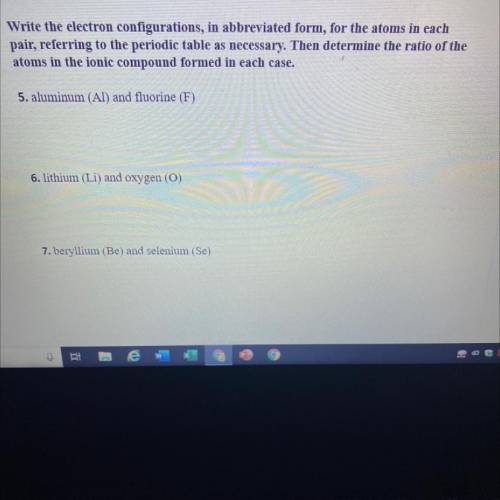
Write the electron configurations, in abbreviated form, for the atoms in each
pair, referring to the periodic table as necessary. Then determine the ratio of the
atoms in the ionic compound formed in each case.
vill
5. aluminum (Al) and fluorine (F)
6. lithium (Li) and oxygen (O)
7. beryllium (Be) and selenium (Se)
PLEASE HELP

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:00
How are ionic bonds formed and what is the attractive force within an ionic bond
Answers: 1

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1
You know the right answer?
Write the electron configurations, in abbreviated form, for the atoms in each
pair, referring to th...
Questions

Computers and Technology, 17.07.2019 12:00

Computers and Technology, 17.07.2019 12:00



Mathematics, 17.07.2019 12:10





English, 17.07.2019 12:10

Mathematics, 17.07.2019 12:10


Mathematics, 17.07.2019 12:10


Health, 17.07.2019 12:10

Geography, 17.07.2019 12:10



Biology, 17.07.2019 12:10



