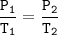Chemistry, 10.12.2020 01:00 chinyere614
The gases in a hairspray can are at a temperature of 27 C and a pressure of 30 psi. if the gases can reach a pressure of 90 psi, the can will explode. To what Temperature must the gases be raised in order for the can to explode? You can assume volume remains constant please help!!!

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:10
Why is the vapor pressure of a warm lake higher than the vapor pressure of a cold lake? o a. warm water has a greater heat of vaporization. ob. warm water evaporates more quickly. cool water evaporates more quickly. od. cool water has a greater heat of vaporization.
Answers: 1

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1
You know the right answer?
The gases in a hairspray can are at a temperature of 27 C and a pressure of 30 psi. if the gases can...
Questions


History, 03.04.2020 03:07


Mathematics, 03.04.2020 03:07


Social Studies, 03.04.2020 03:07

Mathematics, 03.04.2020 03:07

Health, 03.04.2020 03:07


Health, 03.04.2020 03:07

Mathematics, 03.04.2020 03:07


Mathematics, 03.04.2020 03:07


Mathematics, 03.04.2020 03:07

English, 03.04.2020 03:07

Mathematics, 03.04.2020 03:07