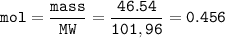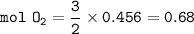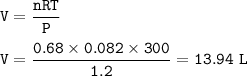Chemistry, 10.12.2020 01:00 haileyhale5
During a laboratory experiment, 46.54 grams of Al2O3 was formed when O2 reacted with aluminum metal at 300.0 K and 1.2 atm. What was the volume of O2 used during the experiment? (5 points) 3O2 + 4Al → 2Al2O3 10.19 liters 11.67 liters 12.51 liters 13.96 liters

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1
You know the right answer?
During a laboratory experiment, 46.54 grams of Al2O3 was formed when O2 reacted with aluminum metal...
Questions


English, 16.07.2021 23:10


Mathematics, 16.07.2021 23:10

Mathematics, 16.07.2021 23:10

Mathematics, 16.07.2021 23:10



Spanish, 16.07.2021 23:20


Mathematics, 16.07.2021 23:20


Mathematics, 16.07.2021 23:20

Mathematics, 16.07.2021 23:20




Computers and Technology, 16.07.2021 23:20