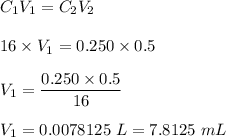Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:20
Which is true of chemicals? a. things containing chemicals always cost a lot of money. b. chemicals are never dangerous. c. chemicals are in many substances in a home. d. chemicals are rarely found on earth.
Answers: 1

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

You know the right answer?
What volume, in mL, of concentrated 16 M nitric acid would you need to use in order to prepare 500.0...
Questions

Mathematics, 23.10.2021 08:20

Mathematics, 23.10.2021 08:20




Mathematics, 23.10.2021 08:20

Social Studies, 23.10.2021 08:20

Mathematics, 23.10.2021 08:20


Social Studies, 23.10.2021 08:20

Mathematics, 23.10.2021 08:30

English, 23.10.2021 08:30

Chemistry, 23.10.2021 08:30


Mathematics, 23.10.2021 08:30


Mathematics, 23.10.2021 08:30



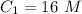 .
.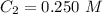 .
.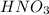 ,
, 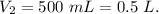
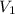 ).
).