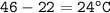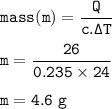Chemistry, 09.12.2020 17:10 jodiconklin2358
A jeweler heated a sample of silver from 22.0°C to 46.0°C. The specific heat of silver is
0.235 J/gºC. If the silver absorbed 26.0 J of heat, what is the mass of the silver?
please help me!

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2


Chemistry, 23.06.2019 06:00
Each step in the following process has a yield of 70% ch4 + 4cl2 yield ccl4 +4hcl ccl4 + 2hf yield ccl2f2 + 2hcl of 4.50 mole ch4 reacts what is the total amount of hcl produced
Answers: 3

You know the right answer?
A jeweler heated a sample of silver from 22.0°C to 46.0°C. The specific heat of silver is
0.235 J/g...
Questions













Mathematics, 20.07.2019 02:10

Mathematics, 20.07.2019 02:10

Advanced Placement (AP), 20.07.2019 02:10



History, 20.07.2019 02:10