
Chemistry, 09.12.2020 02:10 juansebas35
A gas us a pressure of 800 kPa at 327 degree C. What will be its pressure at 27 degree C, if the volume does not change?
A. 400 kPa
B. 1,600 kPa
C. 668 kPa
D. 958 kPa

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1



Chemistry, 23.06.2019 06:00
Complete the sentences to best explain the ranking.match the words below to the appropriate blanks in the sentences.a less polar bondhigher molar massion-dipole forcesstronger intermolecular forcesdipole-dipole forcesdispersion forceshydrogen bonding1. h2s and h2se exhibit the following intermolecular forces:.2. therefore, when comparing h2s and h2se the one with a has a higher boiling point .3. the strongest intermolecular force exhibited by h2o is . therefore, when comparing h2se and h2o the one with has a higher boiling point.
Answers: 1
You know the right answer?
A gas us a pressure of 800 kPa at 327 degree C. What will be its pressure at 27 degree C, if the vol...
Questions


English, 19.08.2021 21:20

Mathematics, 19.08.2021 21:20

Mathematics, 19.08.2021 21:20


Mathematics, 19.08.2021 21:20

English, 19.08.2021 21:20


Mathematics, 19.08.2021 21:20


Mathematics, 19.08.2021 21:20



English, 19.08.2021 21:20






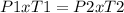 our given variables:P1=800kPa T1= 327 + 273 = 600 KP2= ? (decrease)T2= 27+ 273 = 300 K- rearrange our equation and plug in the numbers to solve.
our given variables:P1=800kPa T1= 327 + 273 = 600 KP2= ? (decrease)T2= 27+ 273 = 300 K- rearrange our equation and plug in the numbers to solve.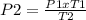
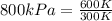 - Our Kelvin cancels out so that leaves us with the measurment of kPa. When the numbers are solved out, you get 1600 kPa
- Our Kelvin cancels out so that leaves us with the measurment of kPa. When the numbers are solved out, you get 1600 kPa

