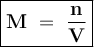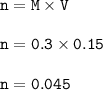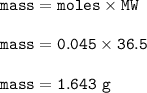Chemistry, 07.12.2020 19:30 Funkyatayo
A solution of HCl is .3M. Determine what mass of acid has been dissolved in 150 mL of solution

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2
You know the right answer?
A solution of HCl is .3M. Determine what mass of acid has been dissolved in 150 mL of solution...
Questions


Mathematics, 28.01.2021 03:10

English, 28.01.2021 03:10

Mathematics, 28.01.2021 03:10

History, 28.01.2021 03:10

Biology, 28.01.2021 03:10



Mathematics, 28.01.2021 03:10


History, 28.01.2021 03:10

Mathematics, 28.01.2021 03:10

Mathematics, 28.01.2021 03:10

Chemistry, 28.01.2021 03:10

Mathematics, 28.01.2021 03:10

Mathematics, 28.01.2021 03:10


Biology, 28.01.2021 03:10

Geography, 28.01.2021 03:10

Mathematics, 28.01.2021 03:10