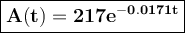Chemistry, 07.12.2020 14:00 lucerogd170
The amount of 217 mg of an isotope is given by A(t) = 217 € -0.0171, where t is time in years since the initial amount of 217 mg was present. Find the amount to the nearest milligraM left after 20 years

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 21.06.2019 17:50
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide
Answers: 2

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2
You know the right answer?
The amount of 217 mg of an isotope is given by A(t) = 217 € -0.0171, where t is time in years since...
Questions

History, 23.08.2019 09:30

Biology, 23.08.2019 09:30


Mathematics, 23.08.2019 09:30

Business, 23.08.2019 09:30

Mathematics, 23.08.2019 09:30

Biology, 23.08.2019 09:30

Mathematics, 23.08.2019 09:30

Mathematics, 23.08.2019 09:30


Mathematics, 23.08.2019 09:30


English, 23.08.2019 09:30

Mathematics, 23.08.2019 09:30





English, 23.08.2019 09:30