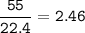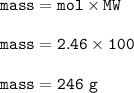Chemistry, 07.12.2020 06:00 nails4life324
When heated, calcium carbonate decomposes to yield calcium oxide and carbon dioxide gas via the reaction
CaCO3(s)→CaO(s)+CO2(g)
What is the mass of calcium carbonate needed to produce 55.0 L of carbon dioxide at STP?
Express your answer with the appropriate units.
mass of CaCO3 =

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1
You know the right answer?
When heated, calcium carbonate decomposes to yield calcium oxide and carbon dioxide gas via the reac...
Questions


Chemistry, 17.04.2020 18:09



Mathematics, 17.04.2020 18:09

History, 17.04.2020 18:09


Mathematics, 17.04.2020 18:09

Social Studies, 17.04.2020 18:09


Mathematics, 17.04.2020 18:09

Mathematics, 17.04.2020 18:09

Mathematics, 17.04.2020 18:09

Mathematics, 17.04.2020 18:09


Mathematics, 17.04.2020 18:09