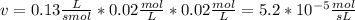Chemistry, 18.08.2019 12:00 emiliapizzillo
The rate law for a hypothetical reaction is rate = k [a][b]. if the concentrations of a and b are both 0.020 moles per liter and k = 1.3 × 10-1 m-1s-1, what is the reaction rate?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 23.06.2019 06:30
Aplanet similar to earth has four moons roughly the same distance away. the moon that will most affect tides on the planet is the one that has the greatest a) mass. b) volume. c) density. d) amount of water.
Answers: 1

Chemistry, 23.06.2019 08:00
Determine the number of moles of air present in 1.35 l at 750 torr and 17.0°c. which equation should you use? n=pv/rt what is the number of moles present? ⇒ 0.056 mol a sample of n2 gas occupying 800.0 ml at 20.0°c is chilled on ice to 0.00°c. if the pressure also drops from 1.50 atm to 1.20 atm, what is the final volume of the gas? which equation should you use? v2= p1v1t2/p2t1 what is the final volume of the gas? ⇒ 932 ml these are the answers
Answers: 1
You know the right answer?
The rate law for a hypothetical reaction is rate = k [a][b]. if the concentrations of a and b are bo...
Questions

English, 29.10.2020 02:00





Mathematics, 29.10.2020 02:00


Mathematics, 29.10.2020 02:00

Mathematics, 29.10.2020 02:00



Mathematics, 29.10.2020 02:00


Mathematics, 29.10.2020 02:00

Mathematics, 29.10.2020 02:00

Mathematics, 29.10.2020 02:00

Mathematics, 29.10.2020 02:00

Mathematics, 29.10.2020 02:00


Mathematics, 29.10.2020 02:00