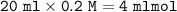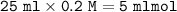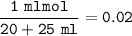Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Aaspirin has a density of 1.40 g/cm^3 what is the volume in cubic centimeters of a tablet weighing 320 mg?
Answers: 3

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1
You know the right answer?
Calculate the pH of the solution that results when 20 mL of 0.2M HCOOH is mixed with 25mL of 0.2M Na...
Questions

Mathematics, 12.08.2020 14:01





World Languages, 12.08.2020 14:01

History, 12.08.2020 14:01

Chemistry, 12.08.2020 14:01

Physics, 12.08.2020 14:01


Computers and Technology, 12.08.2020 14:01

Mathematics, 12.08.2020 14:01

English, 12.08.2020 14:01

Biology, 12.08.2020 14:01


Health, 12.08.2020 14:01




Mathematics, 12.08.2020 14:01