
Chemistry, 06.12.2020 14:00 cthompson1107
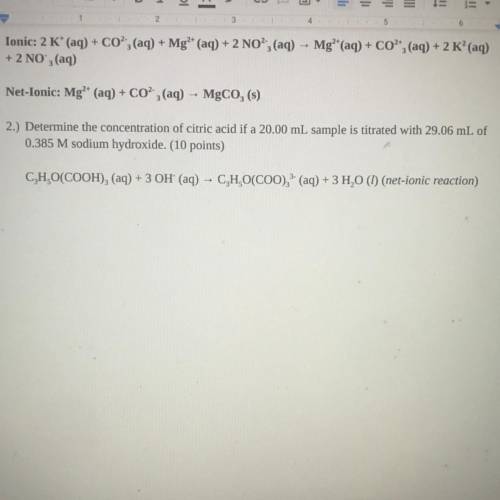
2.) Determine the concentration of citric acid if a 20.00 mL sample is titrated with 29.06 mL of
0.385 M sodium hydroxide. (10 points)
C, H,O(COOH)2 (aq) + 3 OH' (aq) - C, H,O(COO), (aq) + 3 H20 (1) (net-ionic reaction)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1

Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1

Chemistry, 23.06.2019 01:00
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

You know the right answer?
2.) Determine the concentration of citric acid if a 20.00 mL sample is titrated with 29.06 mL of
0....
Questions


Mathematics, 10.04.2020 10:32

Mathematics, 10.04.2020 10:32


Mathematics, 10.04.2020 10:33

Mathematics, 10.04.2020 10:33





Social Studies, 10.04.2020 10:34

Mathematics, 10.04.2020 10:35


Mathematics, 10.04.2020 10:35

Mathematics, 10.04.2020 10:36


English, 10.04.2020 10:36

Biology, 10.04.2020 10:36

Mathematics, 10.04.2020 10:36

Mathematics, 10.04.2020 10:36



