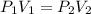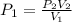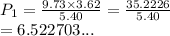A sample of argon, Ar, has a volume of 5.40 L with an unknown pressure. The gas has a volume of 9.73 L when the pressure is 3.62 atm,
with no change in temperature and amount of gas. What was the initial pressue in atm of the gas?
O a. 6.523 atm
O b. 6.52 atm
Oc 2.009 atm
Od. 2,01 atm

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1
You know the right answer?
A sample of argon, Ar, has a volume of 5.40 L with an unknown pressure. The gas has a volume of 9.73...
Questions


Mathematics, 17.03.2022 23:30

Social Studies, 17.03.2022 23:30



Mathematics, 17.03.2022 23:30

Computers and Technology, 17.03.2022 23:30


Mathematics, 17.03.2022 23:40



History, 17.03.2022 23:40

Mathematics, 17.03.2022 23:40




Mathematics, 17.03.2022 23:50



Biology, 17.03.2022 23:50