
Chemistry, 05.12.2020 17:20 ghari112345
For each of the nuclei below write the isotope symbol. Explain how an atom of the unstable isotope carbon -14 becomes an atom of nitrogen 14 if the mass stays the same
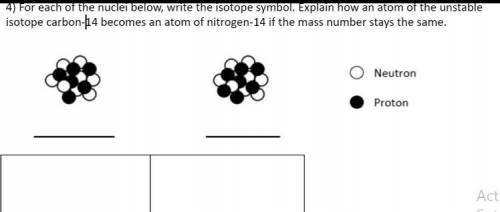

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 23.06.2019 01:00
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1

Chemistry, 23.06.2019 06:30
Aplanet similar to earth has four moons roughly the same distance away. the moon that will most affect tides on the planet is the one that has the greatest a) mass. b) volume. c) density. d) amount of water.
Answers: 1

Chemistry, 23.06.2019 10:00
Compare and contrast an assemblage and a pollen fingerprint by defying both and giving examples of each from the chapter.
Answers: 3
You know the right answer?
For each of the nuclei below write the isotope symbol. Explain how an atom of the unstable isotope c...
Questions




Biology, 06.04.2021 17:50

History, 06.04.2021 17:50

Mathematics, 06.04.2021 17:50


Mathematics, 06.04.2021 17:50

Mathematics, 06.04.2021 17:50

Mathematics, 06.04.2021 17:50








English, 06.04.2021 17:50

Mathematics, 06.04.2021 17:50



