
Chemistry, 05.12.2020 09:20 stephanieanaya7
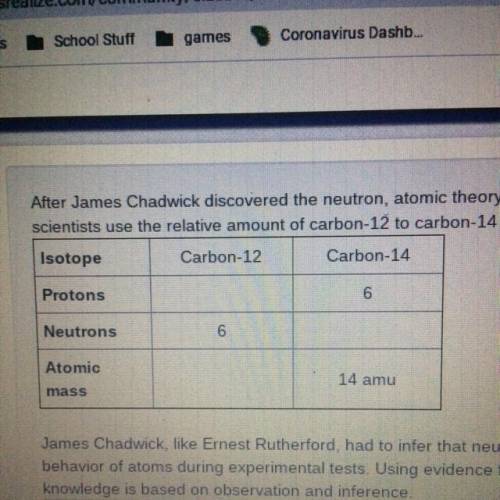
After James Chadwick discovered the neutron, atomic theory expanded to include isotopes. Isotopes have many practical uses. For example,
scientists use the relative amount of carbon-12 to carbon-14 in ancient bones to determine how old they are.
James Chadwick, like Emest Rutherford, had to infer that neutrons were present in atoms. He made inferences based on observations of the
behavior of atoms during experimental tests. Using evidence from Rutherford's gold foil experiment, defend the following claim: Scientific
knowledge is based on observation and inference

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 00:50
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3

Chemistry, 23.06.2019 05:00
Asolution is made by dissolving 2.3 moles of sodium chloride (nacl) in 0.155 kilograms of water. if the molal boiling point constant for water (kb) is 0.51 °c/m, what would be the boiling point of this solution? show all the steps taken to solve this problem.
Answers: 1

Chemistry, 23.06.2019 08:20
At which temperature would a reaction with ah= -220 kj/mol and as=-0.05 kj/(mol-k) be spontaneous?
Answers: 2

Chemistry, 23.06.2019 15:00
20 look at the clock and the data table below. based on the data and on your knowledge of potential and kinetic energy, what is the best conclusion you can make about potential and kinetic energy? the total amount of energy stays the same. the clock has the most potential energy at point b since it is moving the fastest. there is always more potential energy than kinetic energy. potential energy can never be 0, but you can have 0 kinetic energy.
Answers: 1
You know the right answer?
After James Chadwick discovered the neutron, atomic theory expanded to include isotopes. Isotopes ha...
Questions



Mathematics, 01.06.2021 16:50

Mathematics, 01.06.2021 16:50


German, 01.06.2021 16:50



Advanced Placement (AP), 01.06.2021 16:50




Mathematics, 01.06.2021 16:50



English, 01.06.2021 16:50




Mathematics, 01.06.2021 16:50



