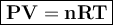Chemistry, 05.12.2020 04:10 alayciaruffin076
A sealed 5.00L flask contains an unknown gas at 1.05 atm and 296 K. How many moles of the unknown gas are in the flask?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Identify the balanced chemical equation that represents a decomposition reaction. p4 + 3o2 ⟶ p2o3 2fe(oh)3 ⟶ 2feo3 + h2o cuso4 ⟶ cuo + 2so3 2fe(oh)3 ⟶ fe2o3 + 3h2o
Answers: 1


Chemistry, 22.06.2019 04:30
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2
You know the right answer?
A sealed 5.00L flask contains an unknown gas at 1.05 atm and 296 K. How many moles of the unknown ga...
Questions

World Languages, 10.11.2020 02:50

Computers and Technology, 10.11.2020 02:50



Biology, 10.11.2020 02:50


Mathematics, 10.11.2020 02:50



Mathematics, 10.11.2020 02:50

Computers and Technology, 10.11.2020 02:50

Mathematics, 10.11.2020 02:50

Physics, 10.11.2020 02:50

Mathematics, 10.11.2020 02:50

Social Studies, 10.11.2020 02:50

Mathematics, 10.11.2020 02:50


Social Studies, 10.11.2020 02:50

SAT, 10.11.2020 02:50

Advanced Placement (AP), 10.11.2020 02:50