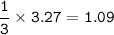Chemistry, 05.12.2020 03:50 nell1234565
How many moles of nitrogen gas would be produced if 3.27 moles of copper(II) oxide were reacted with excess ammonia in the following chemical reaction? 2 NH3(g) + 3 CuO (s) – 3 Cu(s) + N2(g) + 3 H2O(g)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2


Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2
You know the right answer?
How many moles of nitrogen gas would be produced if 3.27 moles of copper(II) oxide were reacted with...
Questions



Chemistry, 10.12.2020 03:00


Mathematics, 10.12.2020 03:00

History, 10.12.2020 03:00




Mathematics, 10.12.2020 03:00

Mathematics, 10.12.2020 03:00



Geography, 10.12.2020 03:00

Mathematics, 10.12.2020 03:00

History, 10.12.2020 03:00


Mathematics, 10.12.2020 03:00

Chemistry, 10.12.2020 03:00

History, 10.12.2020 03:00