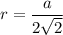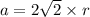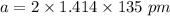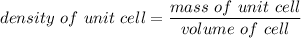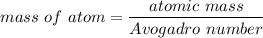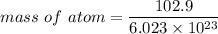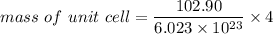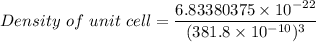Chemistry, 04.12.2020 17:00 SophomoreSareke
Rhodium crystallizes in a face-centered cubic unit cell. The radius of a rhodium atom is 135 pm. Determine the density of rhodium in g/cm3

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3


Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1
You know the right answer?
Rhodium crystallizes in a face-centered cubic unit cell. The radius of a rhodium atom is 135 pm. Det...
Questions

History, 04.06.2021 21:50

Mathematics, 04.06.2021 21:50



Mathematics, 04.06.2021 21:50

Business, 04.06.2021 21:50

Mathematics, 04.06.2021 21:50





Mathematics, 04.06.2021 21:50

Social Studies, 04.06.2021 21:50

Mathematics, 04.06.2021 21:50



Mathematics, 04.06.2021 21:50

Mathematics, 04.06.2021 21:50


Chemistry, 04.06.2021 21:50