
Chemistry, 04.12.2020 17:00 moraaa83egdjr
The reaction between nitrogen and hydrogen to produce ammonia is described by the equilibrium reaction:
3H2 + N2 = 2 NH 3 3H2
What substances are present in the reaction mixture when equilibrium has been obtained.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 22:00
11) burning your hand when accidentally touching a hot plate is an example of which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 2

Chemistry, 23.06.2019 03:00
What do electromagnetic waves carry? how are they produced through which media can they move? where do they transfer energy? what do they not transfer? what do mechanical waves carry? how are they produced? through which media can they move? where do they transfer energy? what do they not transfer?
Answers: 1

Chemistry, 23.06.2019 10:30
Identify the limiting reactant when 9.65-g h2so4 reacts with 6.10-g of naoh.the equation is h2s04 + 2naoh = 2h2o + na2so4• what is the theoretical yield of na2so4, in grams? • how much of the excess reagent will remain after the reaction has been completed? • if 10.5-g of na2so4 are actually recovered experimentally, what is the percent yield?
Answers: 3

Chemistry, 23.06.2019 11:00
The lab procedure involves several factors, listed below some were variable and some were constant. label each factor below v for variable ot c for constant
Answers: 1
You know the right answer?
The reaction between nitrogen and hydrogen to produce ammonia is described by the equilibrium reacti...
Questions

Physics, 18.01.2020 16:31

English, 18.01.2020 16:31



Social Studies, 18.01.2020 16:31

Mathematics, 18.01.2020 16:31



Geography, 18.01.2020 16:31









Geography, 18.01.2020 16:31

Biology, 18.01.2020 16:31

Computers and Technology, 18.01.2020 16:31

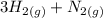 ⇄
⇄ 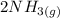
![K = \dfrac{[product]}{[reactants]}](/tpl/images/0949/9170/4abee.png)
![K = \dfrac{[NH_3]^2}{[N_2]^3[H_2]^3}](/tpl/images/0949/9170/fbb32.png)


