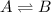Chemistry, 13.01.2020 23:31 chloeethoma24
Which statement correctly defines dynamic equilibrium? at dynamic equilibrium, the rates of forward and reverse reactions are equal. at dynamic equilibrium, the rate of the forward reaction is higher than the rate of the reverse reaction. at dynamic equilibrium, the forward and reverse reactions stop. at dynamic equilibrium, the concentrations of reactants and products are equal.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1
You know the right answer?
Which statement correctly defines dynamic equilibrium? at dynamic equilibrium, the rates of forward...
Questions






English, 25.01.2020 00:31

History, 25.01.2020 00:31

English, 25.01.2020 00:31

Computers and Technology, 25.01.2020 00:31


Physics, 25.01.2020 00:31

History, 25.01.2020 00:31

Computers and Technology, 25.01.2020 00:31

Chemistry, 25.01.2020 00:31

Mathematics, 25.01.2020 00:31


Chemistry, 25.01.2020 00:31


Mathematics, 25.01.2020 00:31