Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

You know the right answer?
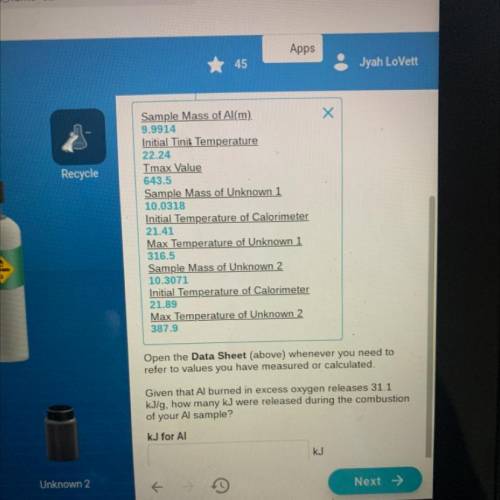
Given that Al burned in excess oxygen releases 31.1 kJ/g how many kJ were released during the combus...
Questions






Mathematics, 23.06.2019 14:10

Mathematics, 23.06.2019 14:10

Chemistry, 23.06.2019 14:10

History, 23.06.2019 14:10


Biology, 23.06.2019 14:10





Mathematics, 23.06.2019 14:10



Physics, 23.06.2019 14:10