
Chemistry, 04.12.2020 01:00 kevonmajor
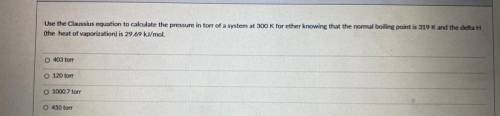
Use the Classius equation to calculate the pressure in torr of a system at 300 K for ether knowing that the normal boiling point is 319 K and the delta H
the heat of vaporization) is 29.69 kl/mol
403 ton
120 ton
1000 7 ore
450 for

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2



Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2
You know the right answer?
Use the Classius equation to calculate the pressure in torr of a system at 300 K for ether knowing t...
Questions

Mathematics, 22.11.2019 03:31















Biology, 22.11.2019 03:31






