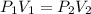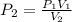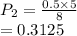Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3


Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3
You know the right answer?
The volume of a gas with an initial pressure of 380 mmHg atm increases from 5.0 L to 8.0 L. What is...
Questions

Social Studies, 18.10.2020 07:01

Mathematics, 18.10.2020 07:01




Physics, 18.10.2020 07:01

Mathematics, 18.10.2020 07:01


Mathematics, 18.10.2020 07:01







Physics, 18.10.2020 07:01


Biology, 18.10.2020 07:01

Physics, 18.10.2020 07:01

History, 18.10.2020 07:01