
Chemistry, 03.12.2020 18:40 rjennis002
The following reaction shows sodium carbonate reacting with calcium hydroxide.
Na2CO3 + Ca(OH)2 → 2NaOH + CaCO3
How many grams of NaOH are produced from 20.0 grams of Na2CO3?
(Molar mass of Na = 22.989 g/mol, C = 12.01 g/mol, O = 15.999 g/mol, Ca = 40.078 g/mol, H = 1.008 g/mol) (5 points)
a. 12.2 grams
b. 15.1 grams
c. 24.4 grams
d. 30.2 grams

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1


Chemistry, 22.06.2019 18:10
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3

Chemistry, 22.06.2019 22:30
What is the work done by the electric force to move a 1 c charge from a to b?
Answers: 2
You know the right answer?
The following reaction shows sodium carbonate reacting with calcium hydroxide.
Na2CO3 + Ca(OH)2 → 2...
Questions

Mathematics, 24.07.2021 16:50





English, 24.07.2021 17:00


English, 24.07.2021 17:00




Mathematics, 24.07.2021 17:00

Computers and Technology, 24.07.2021 17:00




Physics, 24.07.2021 17:00


Mathematics, 24.07.2021 17:00

Mathematics, 24.07.2021 17:00

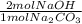 = 0.378 moles NaOH
= 0.378 moles NaOH

