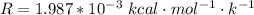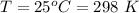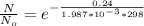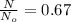Chemistry, 03.12.2020 17:20 eggoysters
1. The thermal interconversion of the axial and equatorial substitutents of the chair conformation of cyclohexane is extremely slow because the two forms are separated by a relatively high activation energy barrier, 10.5 kcal/mol (3672 cm−1). With CN substituents, the equatorial form is only 0.24 kcal/mol (84 cm−1) lower in energy than the axial form. Show how you would determine the ratio between the concentrations of the equatorial and axial forms using the Boltzmann distribution.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 23.06.2019 01:00
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1

Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1

Chemistry, 23.06.2019 02:50
What is the typical rotational frequency frot for a molecule like n2 at room temperature (25∘c)? assume that d for this molecule is 1å=10−10m. take the total mass of an n2 molecule to be mn2=4.65×10−26kg. you will need to account for rotations around two axes (not just one) to find the correct frequency. express frot numerically in hertz, to three significant figures.
Answers: 3
You know the right answer?
1. The thermal interconversion of the axial and equatorial substitutents of the chair conformation o...
Questions



English, 24.01.2022 09:10


Health, 24.01.2022 09:10



Mathematics, 24.01.2022 09:10

Mathematics, 24.01.2022 09:10

Mathematics, 24.01.2022 09:10


Mathematics, 24.01.2022 09:10

English, 24.01.2022 09:10


Mathematics, 24.01.2022 09:10

Mathematics, 24.01.2022 09:10


English, 24.01.2022 09:10

Arts, 24.01.2022 09:10

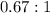
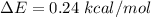
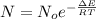
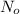 is the number of molecule in equatorial form and N is the number of the molecules in the axial form.
is the number of molecule in equatorial form and N is the number of the molecules in the axial form.