
Chemistry, 03.12.2020 14:00 Lakenwilliams1250
If in the reaction below 32 grams of C2H6 produces 44 grams of CO2, what is the % yield? 2C2H6 + 7O2 = 4CO2 + 6H2O imagine the = is an arrow

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1
You know the right answer?
If in the reaction below 32 grams of C2H6 produces 44 grams of CO2, what is the % yield? 2C2H6 + 7O2...
Questions

Biology, 10.07.2019 02:00


Mathematics, 10.07.2019 02:00

Biology, 10.07.2019 02:00

Mathematics, 10.07.2019 02:00

Social Studies, 10.07.2019 02:00

Mathematics, 10.07.2019 02:00



History, 10.07.2019 02:00

History, 10.07.2019 02:00

Biology, 10.07.2019 02:00

Business, 10.07.2019 02:00

Social Studies, 10.07.2019 02:00

Biology, 10.07.2019 02:00

History, 10.07.2019 02:00

Mathematics, 10.07.2019 02:00

Advanced Placement (AP), 10.07.2019 02:00

Mathematics, 10.07.2019 02:00

History, 10.07.2019 02:00

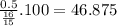 %
%

