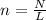Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 21.06.2019 20:00
2h2s + 3o2 2so2 + 2h2o which option gives the correct mole ratios? h2s: so2 = 2: 2 and o2: h2o = 3: 2 h2s: so2 = 2: 3 and o2: h2o = 3: 2 h2s: so2 = 4: 4 and o2: h2o = 5: 4 h2s: so2 = 4: 6 and o2: h2o = 4: 4
Answers: 1

Chemistry, 21.06.2019 20:30
As you move from right to left on the periodic table the atomic radius fill in the blank
Answers: 2

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2
You know the right answer?
How many moles are in a sample of 9.47 x 1024 atoms of argon (Ar)? (Show your calculations for full...
Questions

Mathematics, 30.01.2021 01:00

Mathematics, 30.01.2021 01:00









Mathematics, 30.01.2021 01:00

English, 30.01.2021 01:00



Biology, 30.01.2021 01:00

Biology, 30.01.2021 01:00


History, 30.01.2021 01:00

Mathematics, 30.01.2021 01:00