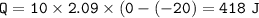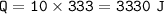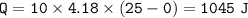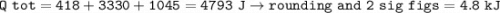Show your work with good use of units, rounding, and significant figures. [Hint: it is good practice to show the value of your answer before you round off to the final answer with the correct significant figures!]
(8 points) How much heat is required to convert 10.00 g of ice at –20.00°C to water at 25°C. The specific heat of ice is 2.09J/g°C; the specific heat of water is 4.182 J/g°C; the heat of fusion is 333.0 J/g.
Group of answer choices

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2

Chemistry, 23.06.2019 01:00
The primary products of complete combustion of fossil fuels are a. carbon dioxide and water b. methane and water c. carbon monoxide and water d. carbon dioxide and carbon monoxide
Answers: 1
You know the right answer?
Show your work with good use of units, rounding, and significant figures. [Hint: it is good practice...
Questions

History, 01.02.2020 02:42

Mathematics, 01.02.2020 02:42

Mathematics, 01.02.2020 02:42



History, 01.02.2020 02:42


Mathematics, 01.02.2020 02:42


Chemistry, 01.02.2020 02:42




History, 01.02.2020 02:42

History, 01.02.2020 02:42


Mathematics, 01.02.2020 02:42


Social Studies, 01.02.2020 02:42