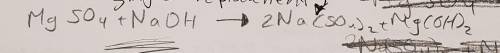Chemistry, 02.12.2020 21:30 irvinbhangal2
3. Magnesium sulfate is added to sodium hydroxide to produce sodium sulfate and magnesium hydroxide (10 points)
a. Write the skeletal equation and Balance the chemical equation describing the reaction above.
b. What kind of reaction is this?
c. Rewrite the balanced chemical equation, adding state symbols for all compounds in the reaction.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1
You know the right answer?
3. Magnesium sulfate is added to sodium hydroxide to produce sodium sulfate and magnesium hydroxide...
Questions

Mathematics, 16.09.2020 22:01

Mathematics, 16.09.2020 22:01

Mathematics, 16.09.2020 22:01

Mathematics, 16.09.2020 22:01

Spanish, 16.09.2020 22:01

Mathematics, 16.09.2020 22:01

English, 16.09.2020 22:01

Mathematics, 16.09.2020 22:01

Mathematics, 16.09.2020 22:01

English, 16.09.2020 22:01

Mathematics, 16.09.2020 22:01

Social Studies, 16.09.2020 22:01

Health, 16.09.2020 22:01

Mathematics, 16.09.2020 22:01

Mathematics, 16.09.2020 22:01

Social Studies, 16.09.2020 22:01

Mathematics, 16.09.2020 22:01

Biology, 16.09.2020 22:01

Mathematics, 16.09.2020 22:01

Mathematics, 16.09.2020 22:01