Chemistry, 02.12.2020 18:30 MrKrinkle77
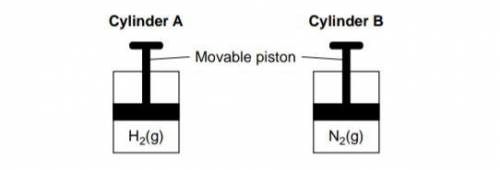
Cylinder A and Cylinder B are sealed, rigid cylinders with movable pistons. Each cylinder contains 500. milliliters of a gas sample at 101.3 kPa and 298 K. Cylinder A contains H2 and cylinder B contains N2. Explain, in terms of collisions between gas molecules and the walls of the container, why pushing the movable piston farther into cylinder B at constant temperature would increase the pressure of the N2 gas.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 14:30
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3

Chemistry, 22.06.2019 23:00
What is the average rate of the reaction between 10 and 20 s?
Answers: 1
You know the right answer?
Cylinder A and Cylinder B are sealed, rigid cylinders with movable pistons. Each cylinder contains 5...
Questions

Geography, 10.11.2020 02:00


History, 10.11.2020 02:00

Mathematics, 10.11.2020 02:00


Mathematics, 10.11.2020 02:00


Mathematics, 10.11.2020 02:00

Engineering, 10.11.2020 02:00



Social Studies, 10.11.2020 02:00

Mathematics, 10.11.2020 02:00

Mathematics, 10.11.2020 02:00


Mathematics, 10.11.2020 02:00

Mathematics, 10.11.2020 02:00



Mathematics, 10.11.2020 02:00